Stainless Steel Grade
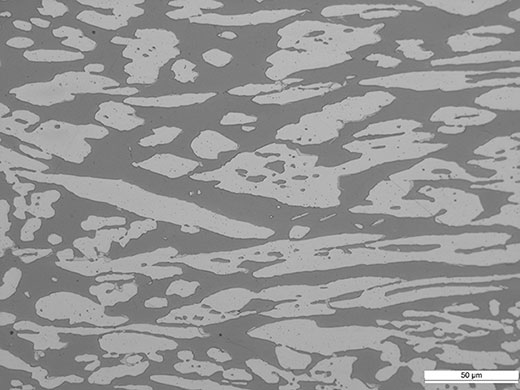 Duplex is a type of chromium-nickel alloy that has a dual composite metal structure by appropriately distributing austenitic and ferritic metal structures.
Duplex is a type of chromium-nickel alloy that has a dual composite metal structure by appropriately distributing austenitic and ferritic metal structures.
Duplex was first commercialized in the early 1930s by Avesta (now Outokumpu) in Sweden.
At that time, due to insufficient refining technology, austenitic steels had high carbon content and often caused intergranular corrosion in various corrosive environments. Duplex was introduced to the European market as a steel grade that compensates for these shortcomings of austenitic steel and was used in sulfite paper mills, explosives factories, etc.
The Korean War that occurred in 1950 ironically promoted the development of duplex research. The war caused an international nickel shortage, which led to the development and stabilization of duplex with relatively lower nickel content compared to austenitic steel. Research results showing that duplex is stronger than austenitic steel against stress corrosion cracking (SCC) in chlorine atmosphere began to be known to the market from this time.
With the development of decarburization equipment (AOD, VOD) in the 1970s, a foundation was laid to finely manage the physical property inhibiting elements of steel including carbon and sulfur.
This also made it possible to finely control the nitrogen content in duplex in addition to carbon and sulfur. Nitrogen is an austenite structure stabilizing element like nickel.
Since it is more economical than nickel, it was first used as a nickel substitute element when making duplex, and because it has great advantages such as improving physical properties, it is currently used as one of the essential elements in duplex composition.
In duplex, nitrogen helps greatly with intergranular corrosion control in the heat-affected zone (HAZ) during welding, in addition to improving physical properties.
Duplex with low nitrogen content has a very fast rate of transformation of the heat-affected zone to a fully ferritic phase during welding. In the fully ferritic phase, the solubility of carbon and nitrogen is very low, so they transform into carbides and nitrides and precipitate at grain boundaries.
Sufficient nitrogen content significantly affected the prevention of sensitization and intergranular corrosion because it delays such intergranular precipitation during duplex welding.
The development and advancement of secondary refining equipment made it possible to finely control the composition of steel, which led to the development of second-generation duplex.
The standard duplex (STANDARD DUPLEX, S32205) steel that we commonly know was developed at this time.
Later, when it was confirmed that elements such as copper (Cu) and tungsten (W) have resistance to pitting, super duplex (SUPER DUPLEX) steel with increased chromium and nickel content and added copper and tungsten was developed,
and lean duplex (LEAN DUPLEX) steel that is economical compared to austenitic steel and has high strength but similar pitting resistance was also developed.
Duplex is steadily increasing in use because it has higher strength and is more economical than austenitic steel and has advantages in various corrosive environments.



